Adiabatic Calorimeter for Comparative Study of Thermal Runaway Hazards in Sodium-ion and Lithium-ion Batteries
Professor Wang Qingsong’s team from the University of Science and Technology of China published a paper titled “Thermal runaway hazards comparison between sodium-ion and lithium-ion batteries using accelerating rate calorimetry” in Process Safety and Environmental Protection (IF: 6.9). This research was jointly supported by the State Key Laboratory of Fire Science at the University of Science and Technology of China and the Energy Storage Research Institute of China Southern Power Grid Company.
The study utilized the BAC-90A small battery adiabatic calorimeter to investigate and compare the thermal runaway characteristics and hazards of three types of 18650 batteries (a sodium-ion battery with NaxTMO2 [NTM] as the cathode and two lithium-ion batteries with LiFePO4 [LFP] and LiNi0.5Co0.2Mn0.3O2 [NCM] as cathodes).

Battery risks are typically assessed based on the triggering conditions and severity of thermal runaway. This study evaluates the thermal runaway hazards of three types of batteries from the following five dimensions, based on experimental data analysis from the BAC-90A small battery adiabatic calorimeter:
- Key parameters of thermal runaway severity: Tmax (maximum temperature during thermal runaway), rmax (maximum temperature rise rate), and W (TNT equivalent).
-
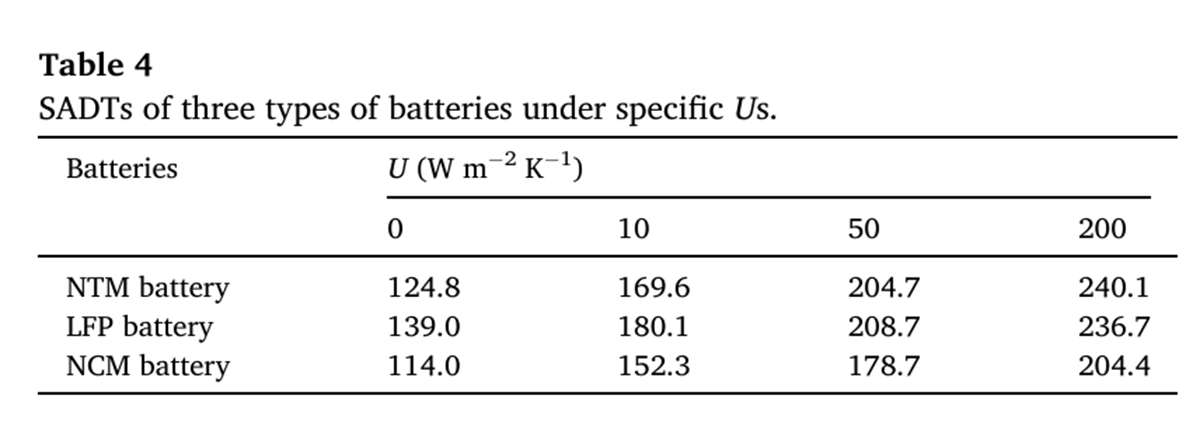
Hazard indicators of self-exothermic critical conditions: Tonset (onset temperature of self-exothermic reaction) and SADTU-2=200WmK-1 (after improving the battery surface heat dissipation conditions). The smaller this value, the more likely the battery is to undergo thermal runaway, and it also reflects the degree to which the battery is affected by heat dissipation conditions. Increasing the SADT by improving the battery surface heat dissipation conditions can help reduce the likelihood of thermal runaway.
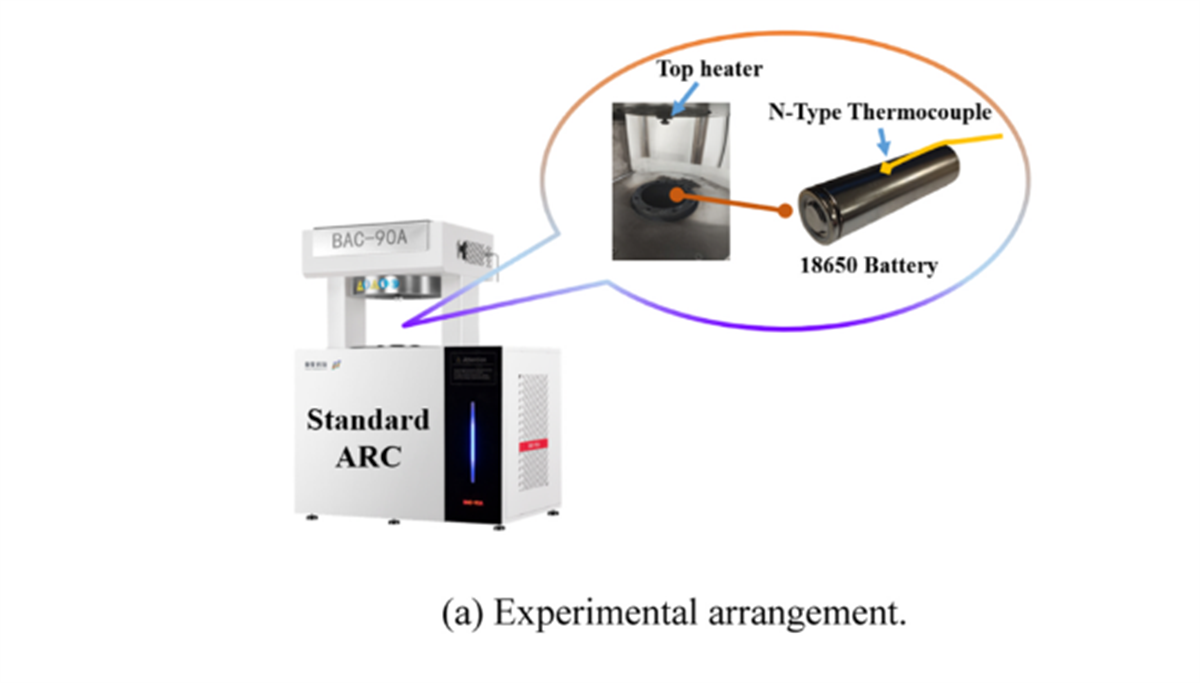
Application of BAC-90A in This Study
- HWS Thermal Runaway Testing
In battery thermal stability research, the HWS (Heating-Waiting-Searching) mode is the most conventional analysis mode. In this study, the HWS mode of the BAC-90A small battery adiabatic calorimeter precisely obtained the characteristic parameters of three types of 18650 batteries, including the onset temperature of self-exothermic reaction (Tonset), the onset temperature of thermal runaway (TTR), the maximum temperature during thermal runaway (Tmax), the maximum temperature rise rate ((dT/dt)max), the depressurization temperature (Topen), and the separator decomposition temperature (Tsc). These parameters serve as the fundamental data for evaluating the thermal characteristics of the three types of batteries.
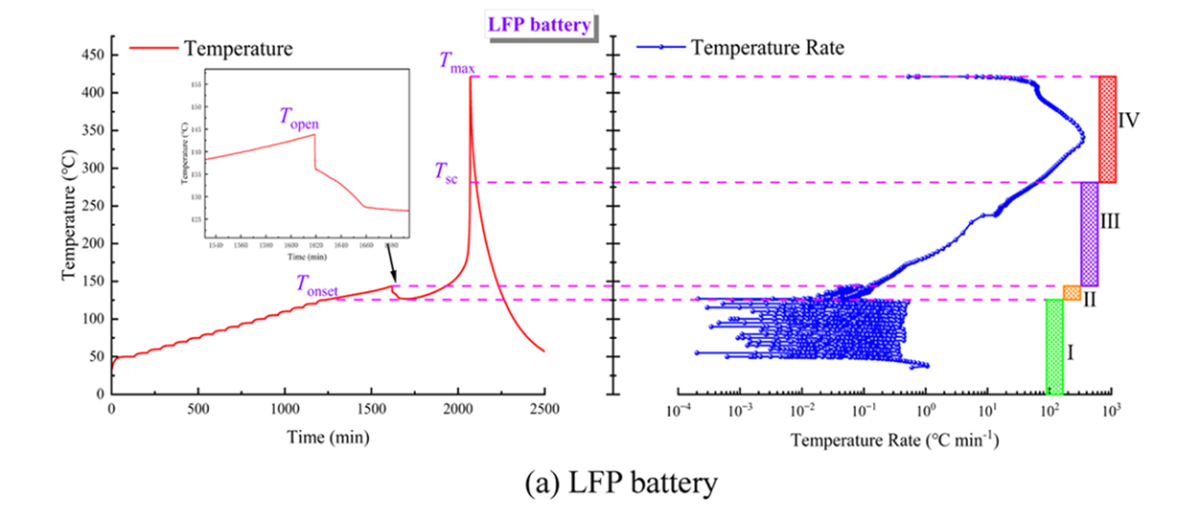
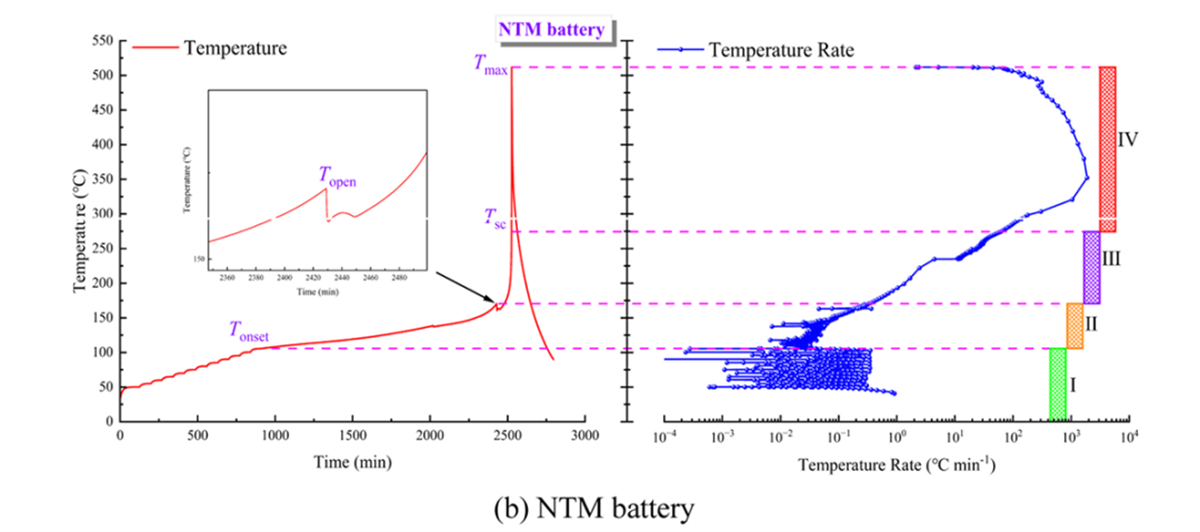
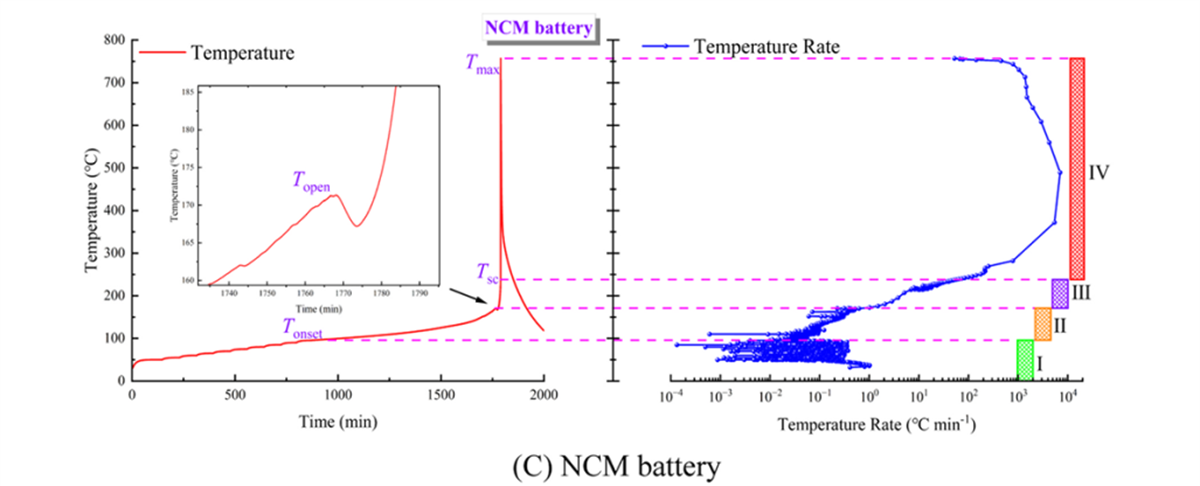
Temperature Changes and Heating Rate Curves for the Three Types of Batteries (Partial Experimental Results Display)
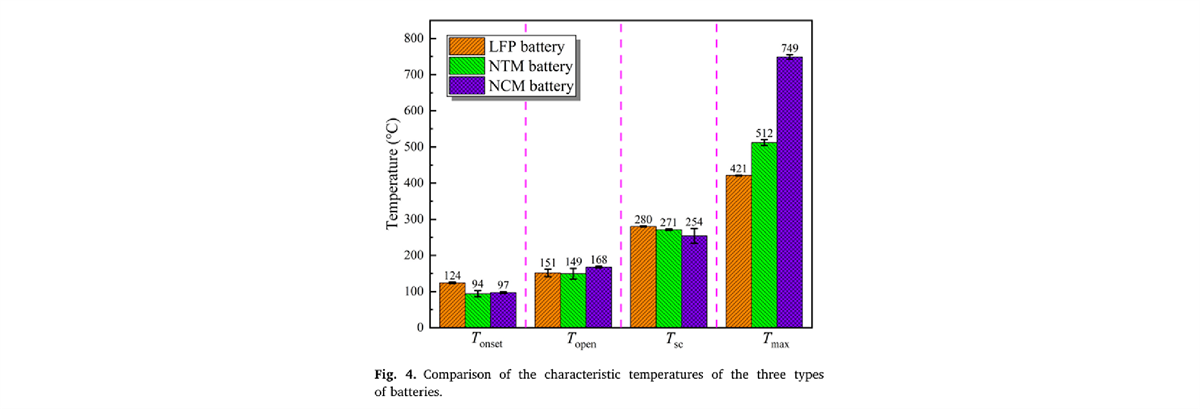
Partial Experimental Results Display
- Thermodynamic Analysis of Self-Heating Reactions
Using the BAC-90A to obtain characteristic parameters of the thermal decomposition reactions of battery materials. By fitting the Arrhenius equation, key kinetic parameters such as pre-exponential factor (A) and activation energy (Ea) of the three types of batteries are derived. It provides a theoretical basis for a deeper understanding of the battery self-exothermic reaction mechanism.
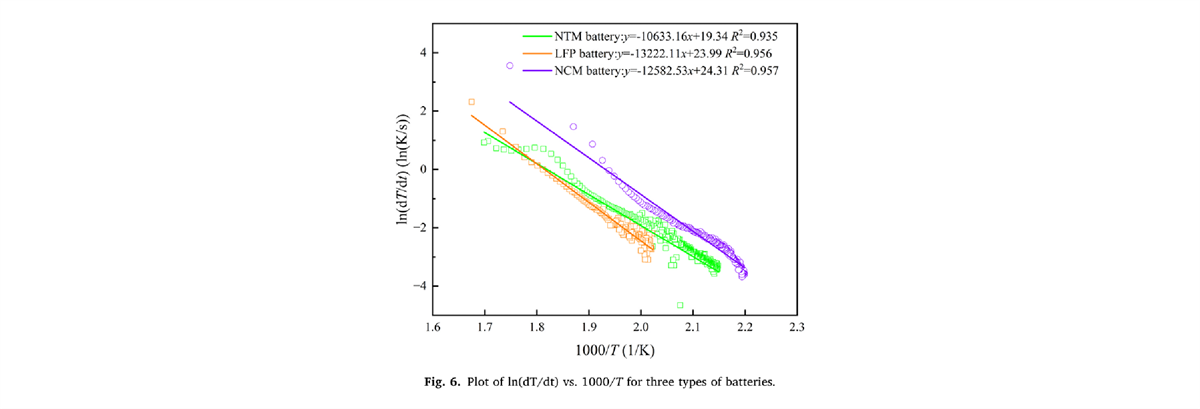
Partial Experimental Results Display
- Calculation of Thermal Runaway Criticality and SADTs
In battery storage and transportation scenarios, when the ambient temperature (T0) rises to a certain level. The system’s heat release rate exceeds the heat dissipation rate, causing the battery temperature to continue to rise until thermal runaway occurs. This critical temperature (T0) is the SADT. This study is based on the thermal characteristic parameters tested by BAC-90A, combined with the thermal balance equation and Semenov model. The logarithmic relationship between the self-accelerating decomposition temperature (SADT) and the system surface heat transfer coefficient (U) of the three types of batteries was calculated. This provides a quantitative basis for assessing the thermal runaway criticality of batteries under different heat dissipation conditions.
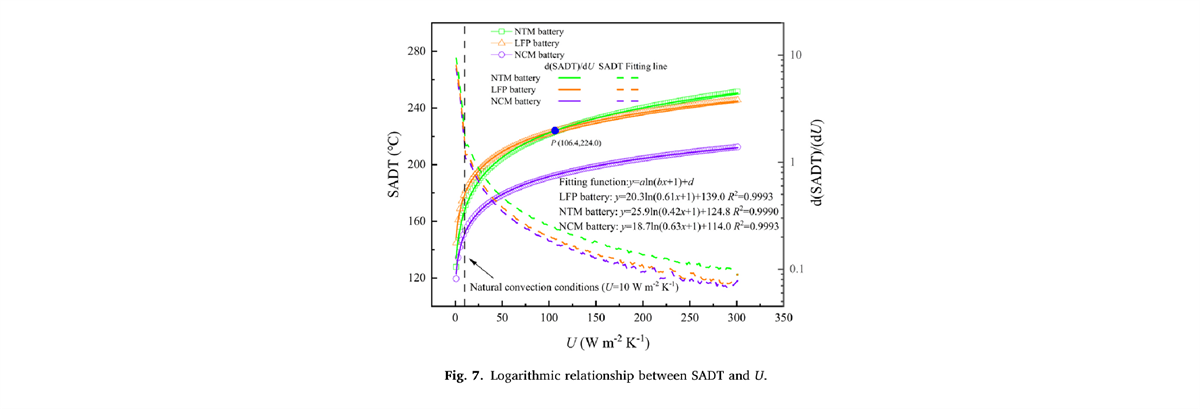
Partial Experimental Results Display
Conclusion
Based on experimental data and analysis results, this study conducted a qualitative assessment of the hazards of the three types of batteries. The conclusions are as follows:
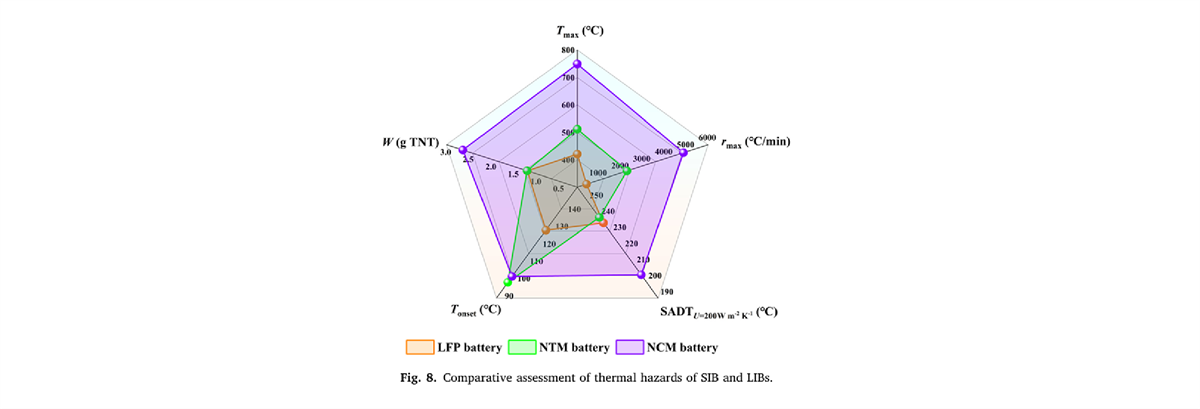
- The maximum temperature during thermal runaway (Tmax) and the maximum temperature rise rate (rmax) for the NTM battery are 511.7℃ and 2285.5℃/min, respectively, which are intermediate between the LFP and NCM batteries.
- The pre-exponential factor (lnA) and activation energy (Ea) for the NTM battery are 13.312 lg/s and 0.8840×105 J/mol, respectively. The TNT equivalent (W) value for the NTM battery is 1.212g TNT, which is almost the same as that of the LFP battery.
- Under natural convection conditions, fully charged NTM batteries will self-ignite when the storage temperature exceeds 169.6℃. Increasing the system’s surface heat transfer coefficient (U) during battery storage and transportation has been proven to significantly enhance the safety of NTM batteries compared to LFP and NCM batteries.
The thermal runaway evaluation model of three types of batteries shows. The order of thermal risk is NCM battery>NTM battery>LFP battery.
Partial Experimental Results Display
This paper provides strong data support for the safety research of sodium-ion batteries and offers significant guidance for hazard assessment. It should be noted that this study only compared and analyzed the thermal runaway hazards of sodium-ion and lithium-ion batteries from a thermodynamic perspective. Subsequent research should further study the composition and amount of toxic and flammable gases produced during battery thermal runaway to more comprehensively evaluate battery safety.