Comparison of Heat of Combustion Test Results for Light Oil Using Different Sampling Methods
The heat of combustion is a core parameter for evaluating the energy quality of fuel oils, and the accuracy of its measurement directly impacts efficiency calculations, trade settlements, and process design. For highly volatile light oils (such as gasoline, naphtha, and jet fuel), selecting an appropriate sealing method for combustion heat testing is a critical factor in obtaining reliable results.
In traditional oxygen bomb calorimeter tests, systematic errors introduced by the sampling method are often overlooked, leading to poor data repeatability and difficulties in comparing results between different laboratories. Improper sampling methods can cause volatile loss during weighing, transfer, and oxygen pressurization, resulting in significantly low measured heat of combustion values.
Therefore, this study, based on the ATC 300AE Oxygen Bomb Calorimeter, systematically compares and analyzes three common sampling methods: the direct sampling method, the adhesive tape sealing method, and the capsule sealing method. The evaluation is conducted from the perspectives of accuracy, precision, and operational convenience in testing the heat of combustion of isooctane, aiming to recommend the optimal sampling solution for light oil combustion heat testing. The test results indicate that the capsule sealing method provides the best repeatability and accuracy, with a relative standard deviation (RSD) of no more than 0.1% and an absolute accuracy within 50 J/g.

Experimental Conditions
Instrument: ATC 300AE Oxygen Bomb Calorimeter
Testing Standard: GB/T 384-2025
Ambient Temperature: 21.2 – 22.5 °C
Consumables: Cotton thread, gelatin capsule, pressure-sensitive adhesive tape (transparent PE)
Sample: Isooctane (AR Grade)
Testing Procedure
Turn on the ATC 300AE Oxygen Bomb Calorimeter.
Step 1: Weigh a certain mass of the sample and either: a) place it directly into the crucible; b) place it in the crucible and seal it with adhesive tape; or c) seal the weighed sample in a gelatin capsule before placing it into the crucible for testing.
Step 2: Connect the ignition wire to the sample using a cotton thread and secure it.
Step 3: Install the oxygen bomb, set the experimental parameters, and enter the sample mass.
Step 4: Initiate the test. The instrument will automatically begin the measurement once the environment is ready.
Step 5: After the test concludes, remove the oxygen bomb, check for complete combustion, and clean it.
Step 6: Repeat the test and record the experimental data.
Experimental Result
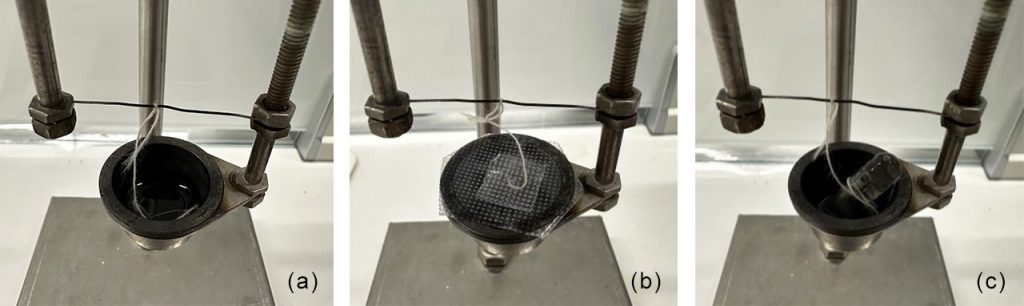
For this experiment, isooctane (standard heat of combustion: 47788 J/g) was selected as the sample to represent highly volatile light oils. The heat of combustion tests were conducted using three different sampling methods: direct sampling, adhesive tape sealing, and capsule sealing, with these sampling configurations illustrated in Figure 2.
The heat of combustion of the sealing materials themselves was calibrated prior to testing: the average heat of combustion of the gelatin capsules was 20647.4 J/g, and that of the pressure-sensitive adhesive tape was 45653.3 J/g. These values were subtracted from the subsequent sample test results. The heat of combustion test results for isooctane using different sampling methods are summarized in Table 1.
| Sampling Method | Direct Sampling | Adhesive Tape Sealing | Capsule Sealing |
| Tape/Capsule Heat of Combustion (J) | / | 4697.9 4889.5 4839.2 | 2089.5 2062.7 2037.9 |
| Sample Mass (g) | 0.5732 0.5711 0.5795 | 0.4728 0.4419 0.4468 | 0.5237 0.5278 0.5252 |
| Sample Heat of Combustion (J/g) | 45698.6 45956.0 45776.2 | 46996.4 46996.5 47326.8 | 47762.5 47790.9 47718.1 |
| Average (J/g) | 45810.3 | 47106.6 | 47757.1 |
| Range (J/g) | 257.4 | 330.3 | 72.8 |
| Accuracy (Deviation from Standard Value) (J/g) | 1977.7 | 681.4 | 30.8 |
| RSD(%) | 0.29 | 0.40 | 0.08 |
The test results indicate that both the direct sampling and adhesive tape sealing methods exhibited varying degrees of sample volatilization between weighing and completion of oxygen bomb assembly. This resulted in a reduction of the actual sample mass participating in combustion, leading to systematically low measured heat of combustion values and poor test repeatability. It should be noted that for the adhesive tape sealing method, part of the mass loss originated from volatilization via the cotton thread in contact with the sample. If this method is used, it is recommended to switch to direct ignition via the ignition wire to reduce this volatilization pathway.
In contrast, the capsule sealing method effectively suppressed sample volatilization and demonstrated good test repeatability, with a relative standard deviation (RSD) of 0.08%, a range of 72.8 J/g, and an average heat of combustion deviating from the standard value by only 30.8 J/g, indicating excellent accuracy and stability.
Regarding operational convenience, both the tape and capsule methods are more complex than direct sampling. Specifically, the tape method requires re-weighing after connecting the ignition wire to the sample before proceeding to connect the ignition wire to the oxygen bomb. This process is cumbersome and introduces numerous uncertainties. The capsule sealing method, effectively eliminating interference from large air bubbles, is less affected by external factors during testing, and its accuracy and stability are superior to those of the tape sealing method.
Experimental Conclusions
This study utilized the ATC 300AE Oxygen Bomb Calorimeter and highly volatile isooctane to systematically compare the effects of three sampling methods on the test results of heat of combustion. The research demonstrates that the capsule sealing method performs optimally in terms of repeatability and accuracy, making it suitable for the precise energy evaluation of highly volatile light oils. These results can provide practical basis and data support for the optimization of relevant testing methods.